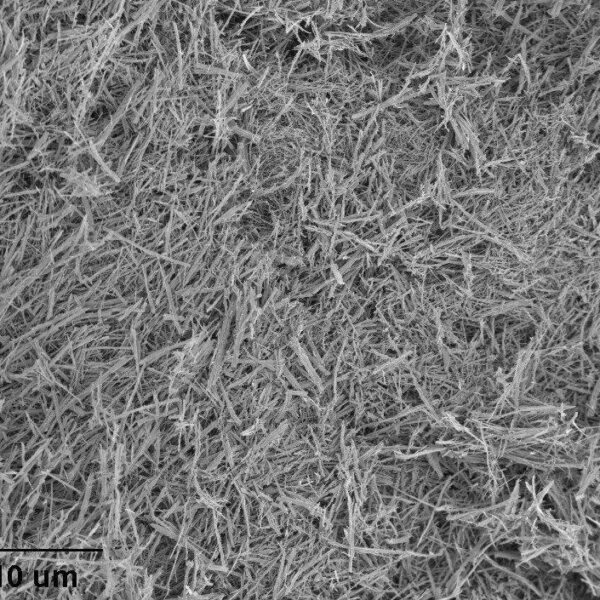
Description
Catalog Number: NovaWire-ATiO-A3-RD
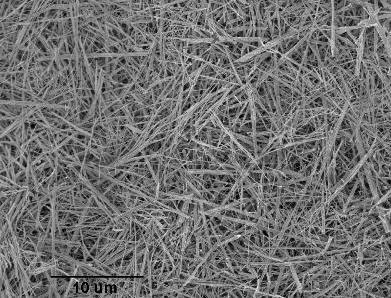
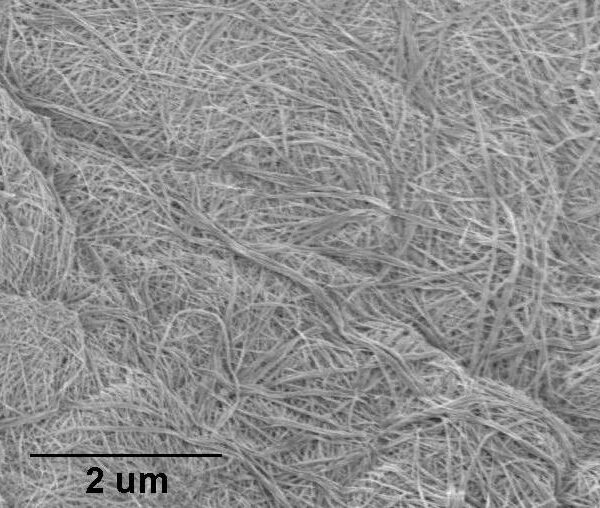
Diameter: ~100nm
Length: ~10µm
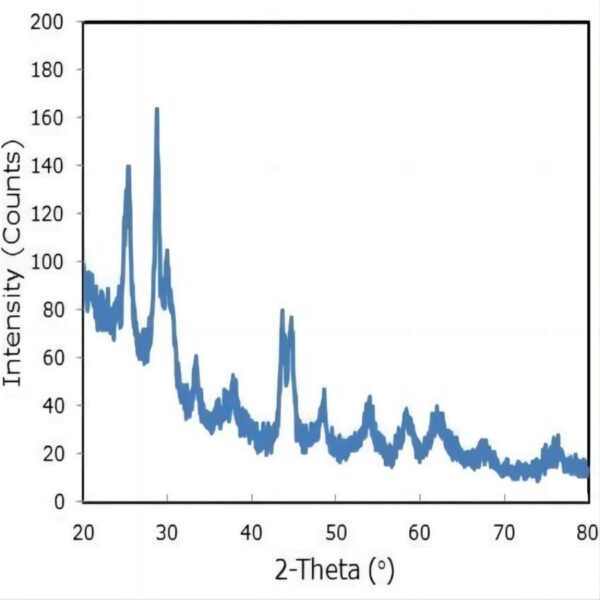
Crystalline Phase: anatase/TiO2(B) mixed-phase
Appearance: dry white powder
APPLICATIONS
The products have unique physicochemical properties, such as non-toxicity, insolubility, high thermal stability and chemical inertness, which ensure their great performance in high temperatures and other harsh environments. And also they are good semiconductors with high photoactivity. Moreover, they have high refractive index and unique ability to reflect light, which allow them to be used in many optical applications, e.g., imparting whiteness, brightness, and opacity to various end-use products.
- Fillers for various nanocomposites including nanowire polymer composites, nanowire metal
composite, and nanowire ceramic composites - Fillers for various adhesives and paints
- Fillers for various high performance films
- Nanowire porous ceramic membranes for chemical and water filtration, which can used in
strong acids and bases - High temperature non-woven textiles
- White pigments for plastics, paints, rubbers, cosmetics, man-made fibers, papers, and
ceramics - Surface coatings
- Catalyst supports
- Photocatalysts
- Dye-sensitized, polymer-based, and quantum dot (QD) solar cells (photovoltaic and
photocatalytic cells) - Water splitting for hydrogen production
- Chemical sensors, especially high temperature gas sensors
- Anodes of lithium ion batteries
- Fuel cells
- Supercapacitors
- Detoxification
- Drug delivery
- Biosensors
- Biocompatible materials for bone implants
- Electrochromic devices
REFERENCES
- In Sun Cho, Chi Hwan Lee, Yunzhe Feng, Manca Logar, Pratap M. Rao, Lili Cai, Dong Rip Kim, Robert Sinclair, Xiaolin Zheng, “Codoping titanium dioxide nanowires with tungsten and carbon for enhanced photoelectrochemical performance”, Nature Communications, 2013, 4, 1723.
- Hoang, S.; Berglund, S. P.; Hahn, N. T.; Bard, A. J.; Mullins, C. B., “ Enhancing visible light photo-oxidation of water with TiO2 nanowire arrays via cotreatment with H2 and NH3: synergistic effects between Ti3+ and N.”, J. Am. Chem. Soc., 2012, 134, 3659.
- Kang, S. H.; Choi, S. H.; Kang, M. S.; Kim, J. Y.; Kim, H. S.; Hyeon, T.; Sung, Y. E., “ Nanorod-Based Dye-Sensitized Solar Cells with Improved Charge Collection Efficiency”, Adv. Mater., 2008, 20, 54.
- Wagemaker, A. P.M. Kentjens, and F. M. Mulder, “Equilibrium lithium transport between nanocrystalline phases in intercalated TiO2 anatase”, Nature, 2002, 418, 397.
- Armstrong, G.; Armstrong, A. R.; Bruce, P. G.; Reale, P.; Scrosati, B., “ TiO2(B) Nanowires as an Improved Anode Material for Lithium-Ion Batteries Containing LiFePO4 or LiNi0.5Mn1.
5O4 Cathodes and a Polymer Electrolyte”, Adv. Mater., 2006, 18, 2597. - Wang, A. Chen, S. H. Jang, H. L. Yip and A. K.-Y. Jen, “Sensitivity of titania(B) nanowires to nitroaromatic and nitroamino explosives at room temperature via surface hydroxyl groups”, J. Mater. Chem., 2011, 21, 7269.
- H. Park, S. Kim, and A.J. Bard, “Novel carbon-doped TiO2 nanotube arrays with high aspect ratios for efficient solar water splitting”, Nano Letters, 2006, 6, 24.
- Zeng, T.-W.; Lin, Y.-Y.; Lo, H.-H.; Chen, C.-W.; Chen, C.-H.; Liou, S.-C.; Huang, H.-Y.; Su, W.-F., “A large interconnecting network within hybrid MEH-PPV/TiO2 nanorod photovoltaic devices”, Nanotechnology, 2006, 17, 5387.
- Lin, Y. Y.; Chu, T. H.; Li, S. S.; Chuang, C. H.; Chang, C. H.; Su, W. F.; Chang, C. P.; Chu, M. W.; Chen, C. W., “ Interfacial Nanostructuring on the Performance of Polymer/TiO2 Nanorod Bulk Heterojunction Solar Cells”, J. Am. Chem. Soc., 2009, 131, 3644.
- Wang, Q.; Wen, Z. H.; Li, J. H., “A Hybrid Supercapacitor Fabricated with a Carbon Nanotube Cathode and a TiO2–B Nanowire Anode”, Adv. Funct. Mater. 2006, 16, 2141.
- Francioso, A. Forleo, A. M. Taurino, and P. Siciliano, “Nanofabrication of TiO2 nanowires: I-V characteristic and improvement of metal oxides gas sensing properties”, Proc. SPIE 6589, Smart Sensors, Actuators, and MEMS III, 2007, 658913.
- S. Mandal, and A. J. Bhattacharyya, “Titania nanowires as substrates for sensing and photocatalysis of common textile industry effluents”, Talanta, 2010, 82, 876.
- Chauhan, S. Chattopadhyay, and P. Mohanty, “Fabrication of titania nanowires
incorporated paper sheets and study of their optical properties”, Materials Express, 2013, 3, 343. - Ru-Hua Tao, Jin-Ming Wu, Hong-Xing Xue, Xiao-Mei Song, Xu Pan, Xia-Qin Fang, X. D. Fang, and S. Y. Dai, “A novel approach to titania nanowire arrays as photoanodes of back-illuminated dye-sensitized solar cells”, Journal of Power Sources, 2010, 195, 2989.
- X. Liu, D. Z. Yang, F. Shi, and Y. J. Cai, “Sol-gel deposited TiO2 film on NiTi surgical alloy for biocompatibility improvement,” Thin Solid Films, 2003, 429, 225.
- Giavaresi, L. Ambrosio, and L. Ambrosio, “Histomorphometric, ultrastructural and microhardness evaluation of the osseointegration of a nanostructured titanium oxide coating by metal-organic chemical vapour deposition: an in vivo study,” Biomaterials, 2004, 25, 5583.
- Burschka, J.; Pellet, N.; Moon, S. J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M. K.; Gratzel, M., “Sequential deposition as a route to high-performance perovskite-sensitized solar cells”, Nature, 2013, 499, 316.
SYNONYM
Anatase nanowires, anatase nanofibers, titanium oxide nanowires, titanium oxide nanofibers, titanium oxide, titania nanowires, titania nanofibers, titania, titanium dioxide nanofibers, titanium dioxide nanowires, titanium dioxide, titanium(IV) oxide nanowires, titanium(IV) oxide nanofibers, titanium(IV) oxide, TiO2 nanowires, TiO2 nanofibers, TiO2, anatase nanowire, anatase nanofiber, titanium oxide nanowire, titanium oxide nanofiber, titania nanowire, titania nanofiber, titanium dioxide nanofiber, titanium dioxide nanowire, titanium(IV) oxide nanowire, titanium(IV) oxide nanofiber, TiO2 nanowire, TiO2 nanofiber